A 48-year-old male presented with a history of chest pain during exertion over the past ten days. On admission, he experienced severe chest pain, rated at 9 out of 10 in intensity.
He has no known comorbidities, does not smoke, and has no family history of coronary artery disease (CAD).
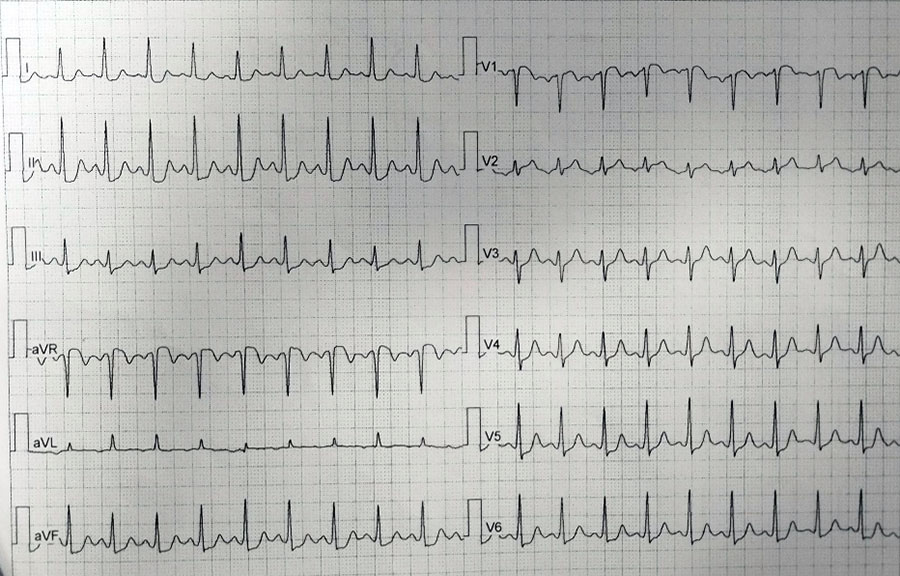
Initial EKG revealed significant abnormalities, including ST depression in leads V1 to V5 and negative T waves in leads D1 and aVL


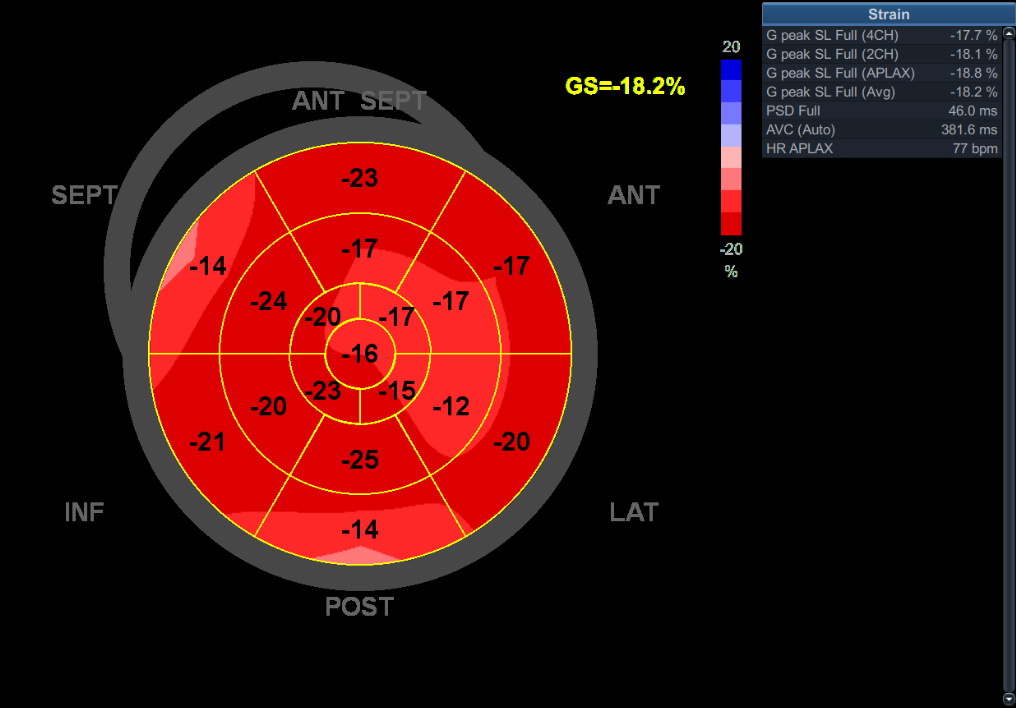
Transthoracic EHO on admission: globular left ventricle, hypokinesis of the inferolateral wall. Mitral regurgitation 2+
Given the severity of his symptoms and the EKG findings, an urgent referral to the catheterization lab was made.
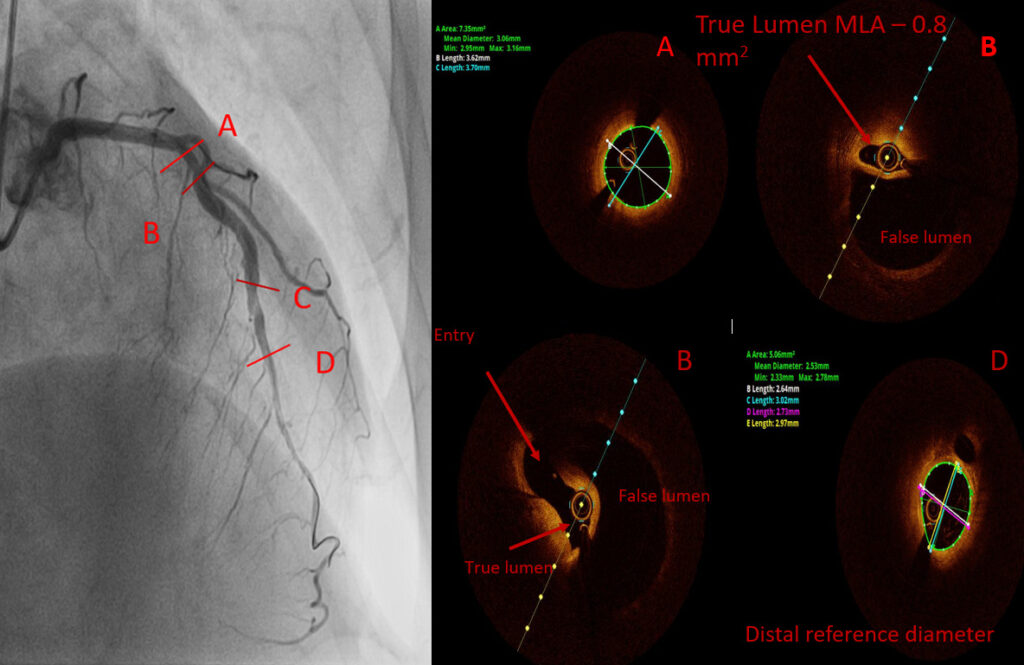
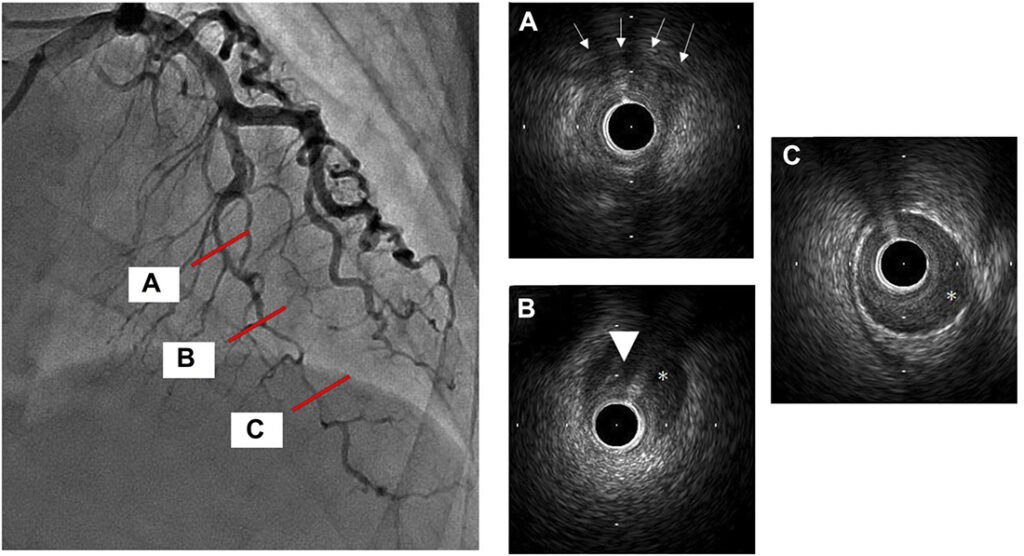
Coronary angiogram:
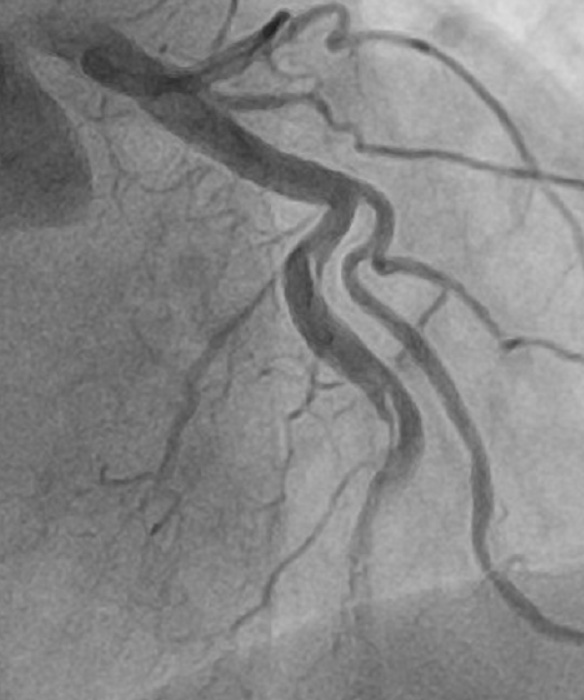
A medial occlusion of the left circumflex (LCx) artery was discovered.
Notably, the left anterior descending (LAD) and right coronary artery (RCA) were free of disease.
Immediate percutaneous coronary intervention was performed.
Predilatation was performed using a 2.5x15mm semi-compliant balloon

Subsequently, a 4.5x16mm drug-eluting stent was successfully implanted at the site of the occlusion
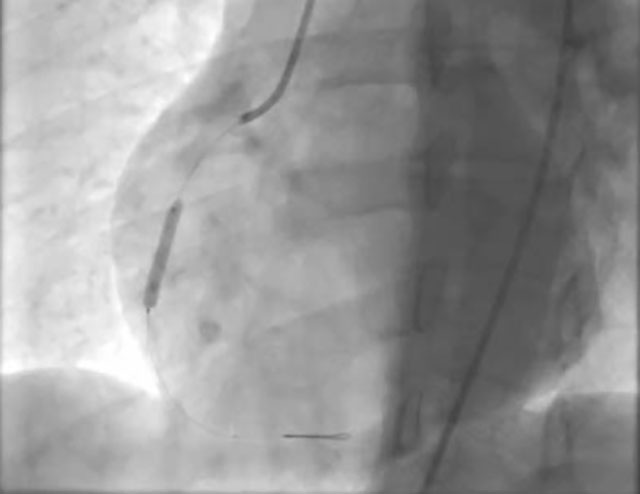
However, post-stent implantation, it was observed that the occlusion appeared to have shifted distally.
This posed a new challenge, raising several critical questions about the next steps in the patient’s treatment.
To gain a better understanding of the situation, intravascular ultrasound (IVUS) was performed.
IVUS revealed the presence of an intramural hematoma, which explained the distal shift of the occlusion.

A decision was made to implant a second stent and a 4.0x20mm DES was placed successfully
Although the hematoma shifted distally once again, blood flow was preserved.
The patient remained stable throughout the procedure and during the post-operative period
He was discharged with the following therapy:
Dual Antiplatelet Therapy (DAPT)
High-intensity statin
Bisoprolol
Ezetimibe
Proton Pump Inhibitor (PPI)
Author: Dr Željko Živanović i Doc. Dr Bojan Stanetić
What did happen at this procedure?















 ECG during chest pain:
ECG during chest pain:


 DES 3.50×30 mm @ 12atm.
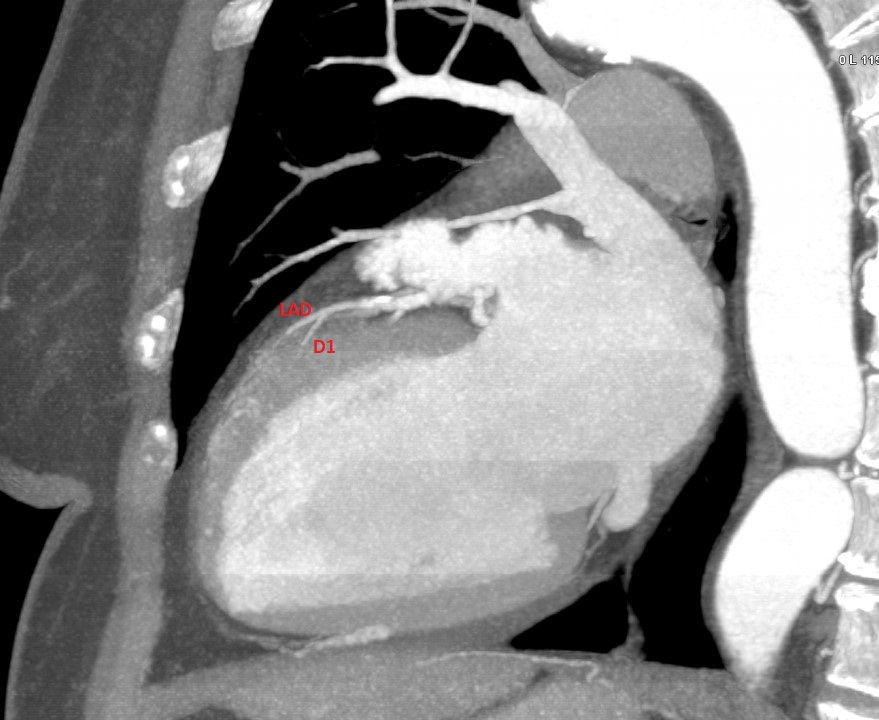
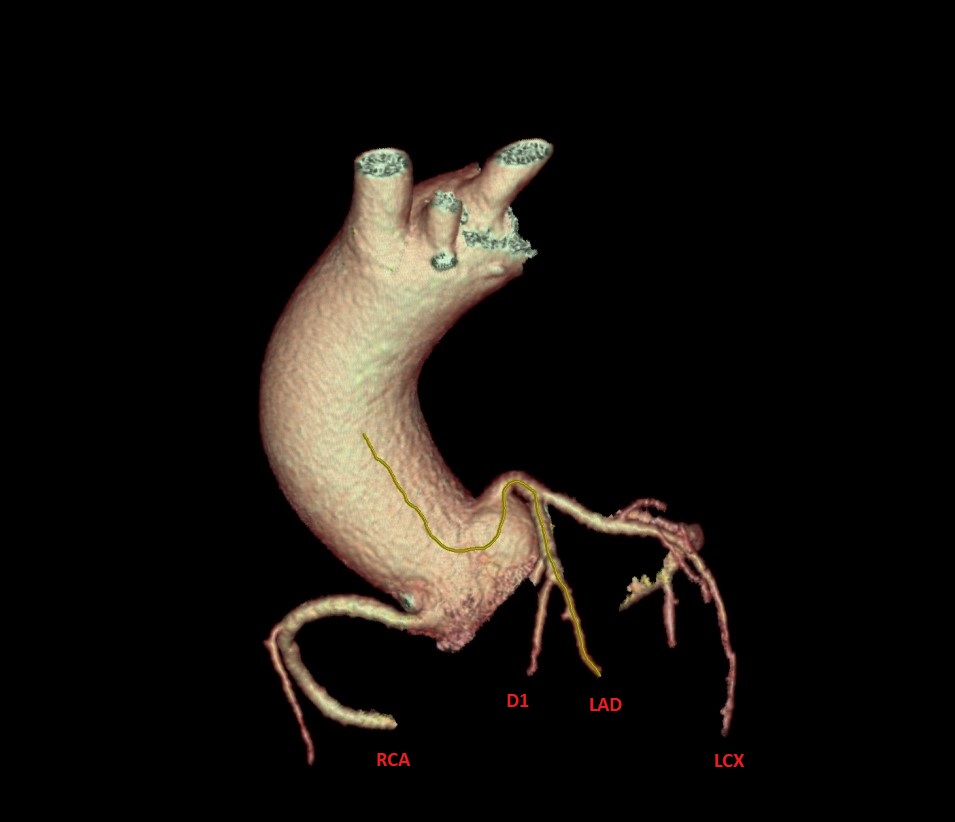
DES 3.50×30 mm @ 12atm. After initial stabilisation patient was transferred to the tertiary center for coronary angiography and further diagnostic.
After initial stabilisation patient was transferred to the tertiary center for coronary angiography and further diagnostic.




 Risk factors for CVD: hypertension, obesity (BMI 36), positive family history of CVD
Risk factors for CVD: hypertension, obesity (BMI 36), positive family history of CVD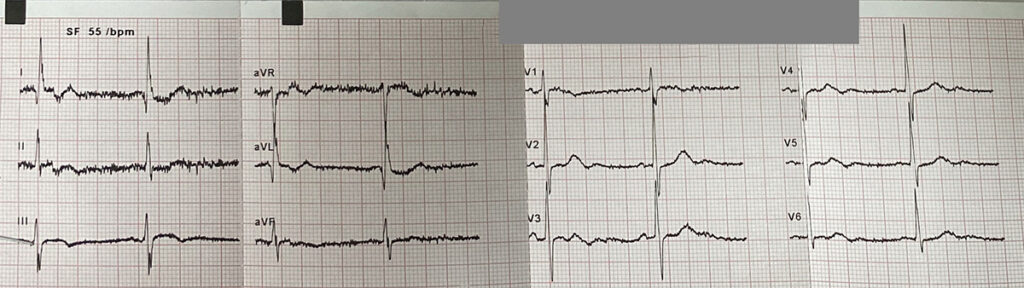


 LAB: TnI 2.180; K 3.1; Hol 4.47; LDL 2.49; HDL 1.24; TRIG 1.6
LAB: TnI 2.180; K 3.1; Hol 4.47; LDL 2.49; HDL 1.24; TRIG 1.6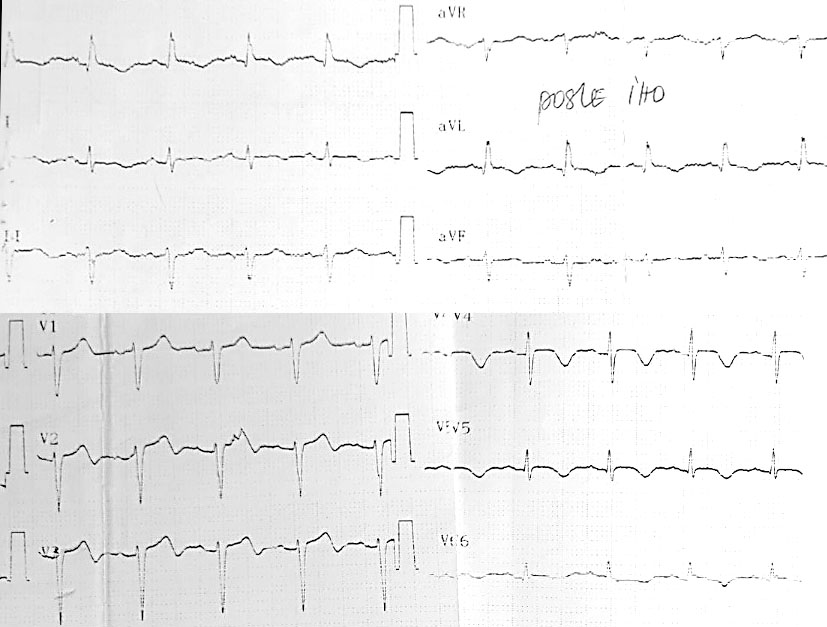 ECHO day one:
ECHO day one:








Great images of the case! Since patient was without chest pain, with a TIMI 2-3 flow in LAD, would opt for conservative treatment (no PCI, no DAPT or LMWH, control angio before dismission). Best regards for colleagues from IKVB Sremska Kamenica!
It is a really great case. I am not an interventional cardiologist, however, it is very illustrative. And what did you do? Could you inform us about the FU of this patient, please?
Thank you for these experience.
I would also stay to a conservative approach.
How do you explain the association of aortic dissection and SCAD?
What do you think about contrast retension before LM? Calcium?
Very interesting case. Of curse conservative approach is correct choice for this patients. Fibromuscular dysplasia can be pathophysiological mechanism who can explain aortic dissection and SCAD. Shadow (contrast retention) before LM is similar like calcium, but must think about aortic dissection especially in patients with history for aortic dissections and SCAD!
Since the patient had NSTEMI complicated with VF and OHCA I wouldn’t be brave enough to proceed with conservative treatment without a further diagnostic.
I would do intracoronary imaging to assess the distribution and MLA of the true lumen. If the true lumen is severely compromised I would proceed with PCI. I agree with Dr Mitov that FMD can be the cause.